Preparation Of Hexaamminenickel Ii Chloride Pdf

11 calculate the percent by mass of nh3 in the three samples.
Preparation of hexaamminenickel ii chloride pdf. 10 calculate the mass of nh3 molecular weight 17 03 g mol in the complex salt in each flask. Hexaamminenickel ii h18n6ni 2 cid 6857628 structure chemical names physical and chemical properties classification patents literature biological. Pubchem substance id. Linear formula ni nh 3 6 cl 2.
This is a brief version of a lab writeup i recently performed. Mostly putting it here as i found it interesting and because really chemistry is bloody awesome. Ive made this with copper sulfate before forming tetraaminecopper ii sulfate. 12 calculate the mean and standard deviation of the three results in step 11.
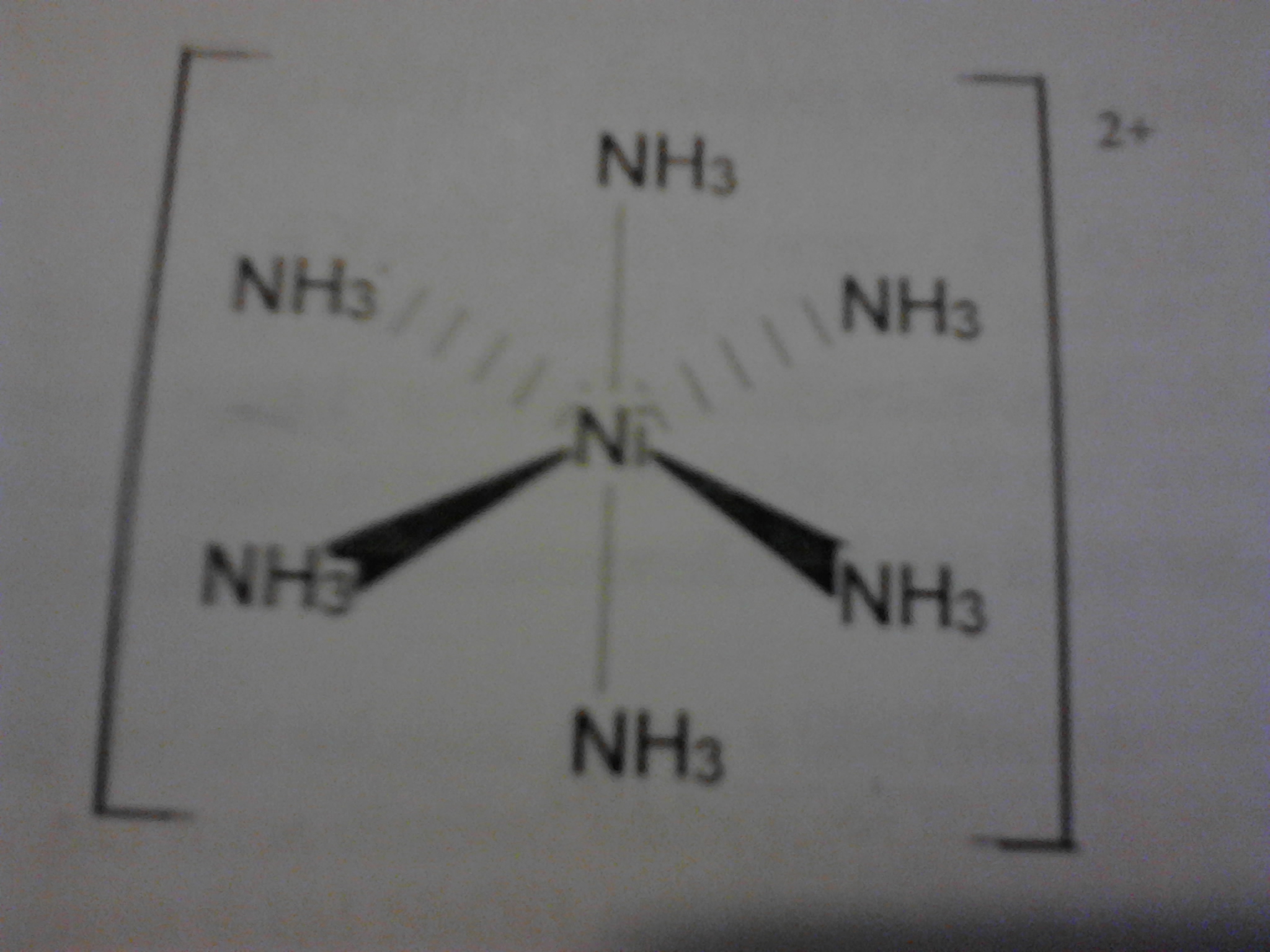
255807 sigma aldrich hexaamminenickel ii chloride 99 999 cas number 10534 88 0. But i love the color. 2 is called hexamminenickel ii ion. Academia edu is a platform for academics to share research papers.
I found the best way to get a good yield was to filter and dry it asap. In the laboratory 1 use an analytical balance to tare a weighing dish and then weigh out anywhere from 4 0 to 4 5 g of nickel chloride. Hexaamminenickel chloride is the chemical compound with the formula ni nh 3 6 cl 2 it is the chloride salt of the metal ammine complex ni nh 3 6 2 the cation features six ammonia called ammines in coordination chemistry ligands attached to the nickel ii ion. High purity submicron and nanopowder forms may be considered.
Hexaamminenickel ii chloride is generally immediately available in most volumes. Preparation of hexamminenickel ii chloride. Properties and structure ni nh 3 6 2 like all octahedral nickel ii complexes is paramagnetic with two unpaired electrons. Posted on september 25 2013 by packetforger.
Treating an iodide with nickel dioxide and sulfuric acid sublimes the iodine. Oooh i like this. In this experiment we will synthesize the complex salt hexamminenickel ii chloride. Molecular weight 231 78.
In the next experiment we will analyze for the percent ammonia in this salt. 255807 hexaamminenickel ii chloride email this page to a friend. Iodide compounds are used in internal medicine.