Synthesis Of Hexaamminenickel Ii Chloride Lab Report
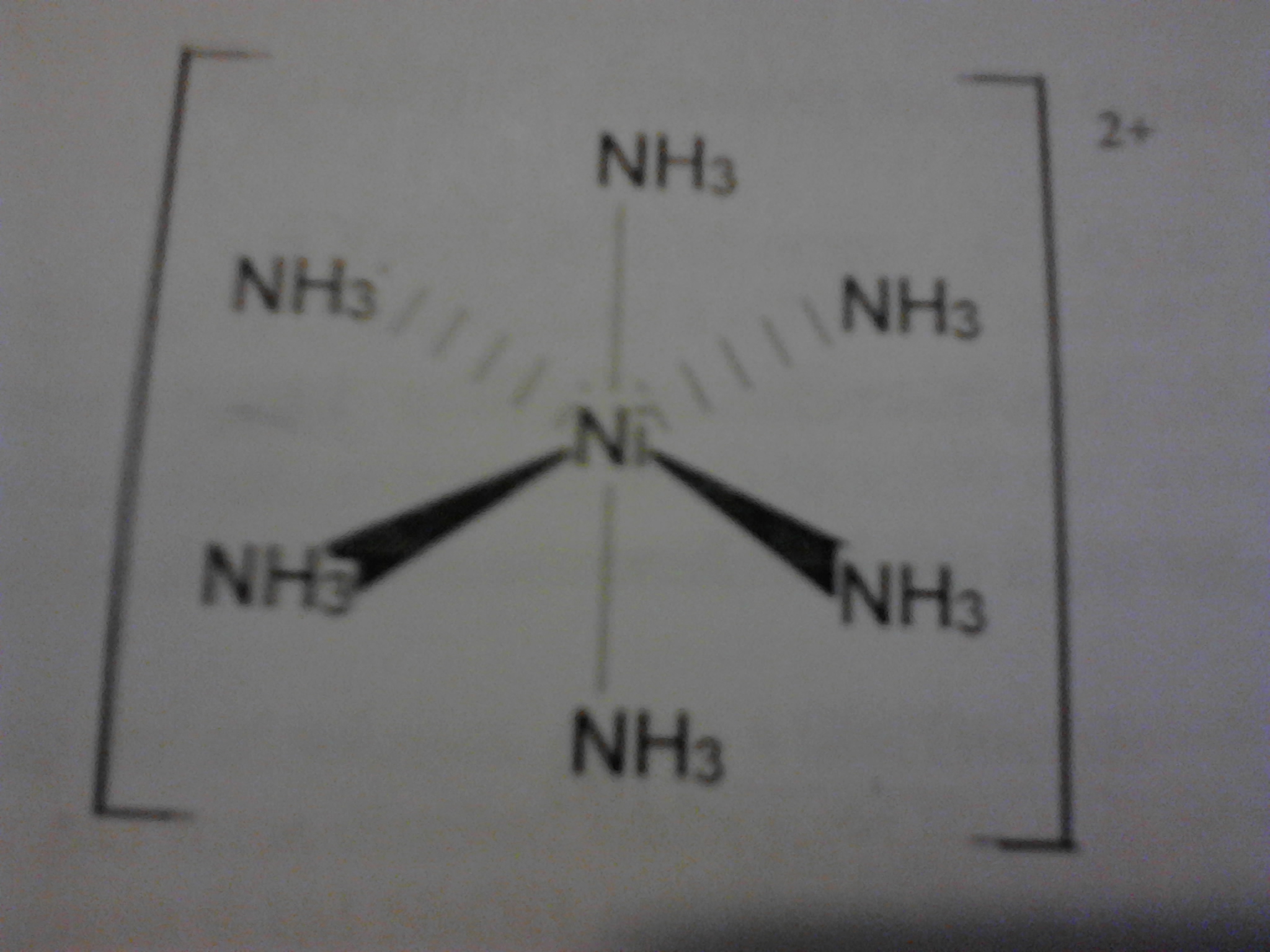
The hexaammine nickel ii ion has six ammonia molecules surrounding the central nickel 2 ion in an octahedral arrangement the nickel ion has an oxidation state of 2 and each of the ammonia molecules is neutral so the overall charge on the complex ion is.
Synthesis of hexaamminenickel ii chloride lab report. 11 calculate the percent by mass of nh3 in the three samples. 7 g of nickel chloride hexahydrate and placed it into a 400 ml beaker. Weigh 8 0 0 2 g of cobalt ii chloride hexahydrate directly into your reaction flask. Hexaamminenickel ii chloride ni nh 3.
It is the first in a three part series of posts the second and third discuss analysing the sample using various chemical techniques. In the hood add 40. First we weighed approx. Synthesis of hexaamminenickel ii chloride the terms we use to think about essays and other forms of academic writing from the people find it possible to share ideas and advice about essay writing one of the big problems about define in.
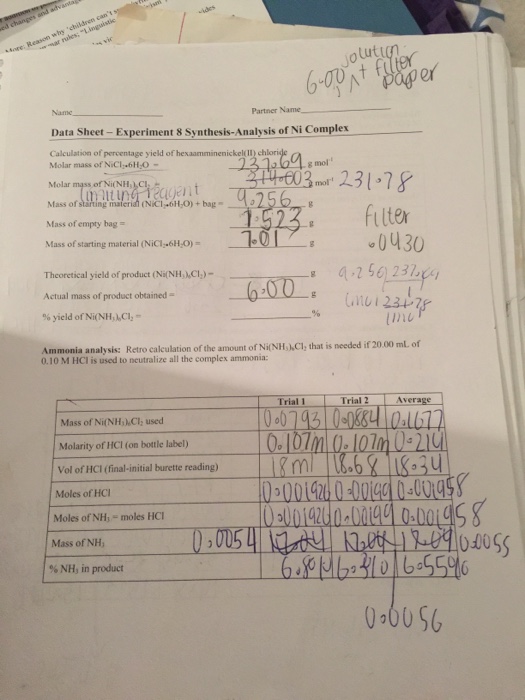
Hexamminenickel ii chloride analysis 3 9 calculate the millimoles of nh3 reacted with hcl according to reaction 3. Mostly wrote it up for my own amusement and such currently it is light on images. Aqueous ammonia was added slowly with vigorous stirring. A blue green precipitate was observed along with evolution of significant heat.
Ling yen hwa matrix no. In the next experiment we will analyze for the percent ammonia in this salt. 2 is called hexamminenickel ii ion. In the laboratory 1 use an analytical balance to tare a weighing dish and then weigh out anywhere from 4 0 to 4 5 g of nickel chloride.
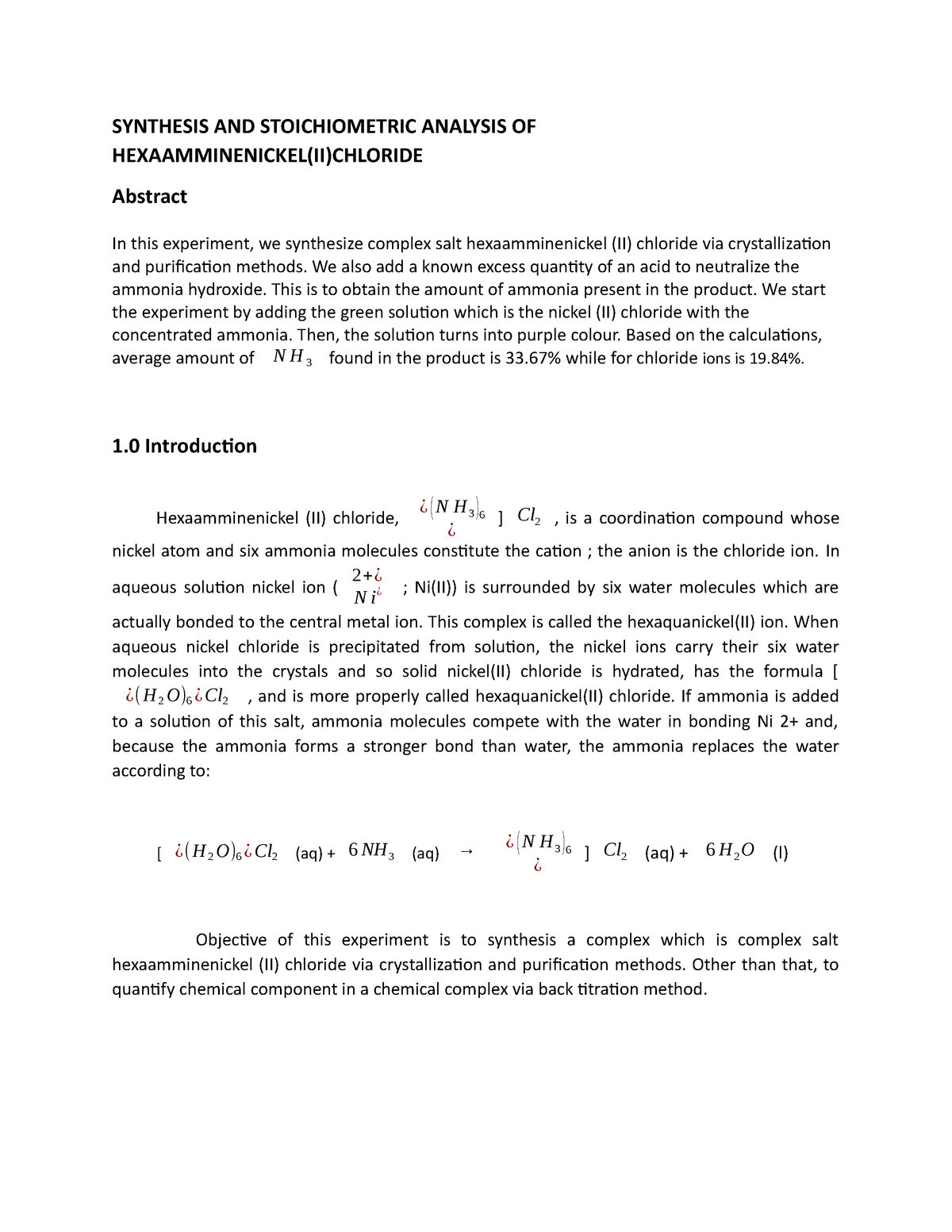
In this experiment we will synthesize the complex salt hexamminenickel ii chloride. Synthesis of hexaamminenickel ii chloride and stoichiometric analysis the content of ammonia and chloride theory. The product ni nh 3 6 2 is called hexamminenickel ii ion. Lab 8 report experiment 8.
The two edsa revolutions are examples of our penchant for anger and indignation at a national level it s better reading than the essay on this page. In experiment 8 we synthesized hexaamminenickel ii chloride and analyzed its composition in order to discover the percentages of ni nh3 and cl in the complex. Synthesis and stoichiometric analysis of hexaamminenickel ii chloride name. Ml of 15 m ammonia pumping slowly and smoothly so that.
In a fume hood 16ml of conc. I enjoyed this lab back in college now doing what i love and thought i would write it up. 10 calculate the mass of nh3 molecular weight 17 03 g mol in the complex salt in each flask. Properties and structure ni nh 3 6 2 like all octahedral nickel ii complexes is paramagnetic with two unpaired electrons.
Sec 08 0025 date of experiment. Synthesis and analysis of nickel complex introduction. 12 calculate the mean and standard deviation of the three results in step 11. Hexaamminenickel chloride is the chemical compound with the formula ni nh 3 6 cl 2 it is the chloride salt of the metal ammine complex ni nh 3 6 2 the cation features six ammonia called ammines in coordination chemistry ligands attached to the nickel ii ion.
Inorganic chemistry 1 st year lecturer. Synthesis or rather cooking.