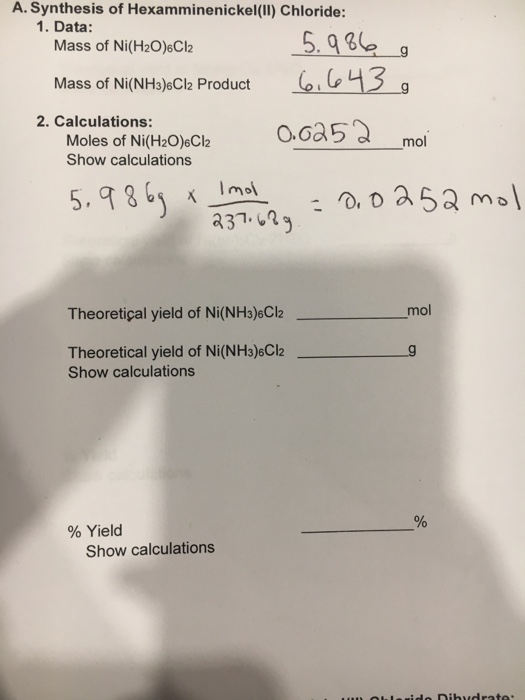Synthesis Of Hexaamminenickel Ii Chloride
Weigh 8 0 0 2 g of cobalt ii chloride hexahydrate directly into your reaction flask.
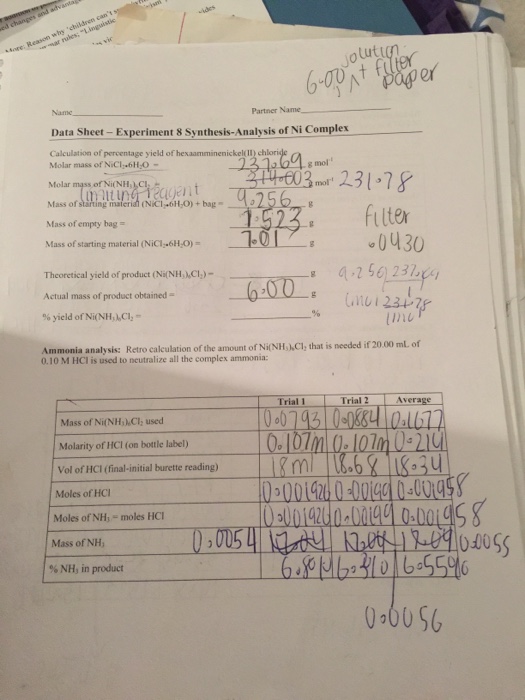
Synthesis of hexaamminenickel ii chloride. Synthesis or rather cooking. 11 calculate the percent by mass of nh3 in the three samples. Find sigma aldrich 255807 msds related peer reviewed papers technical documents similar products more at sigma aldrich. Hexamminenickel ii chloride synthesis 2 go to a fume hood for the next step.
Academia edu is a platform for academics to share research papers. I found the best way to get a good yield was to filter and dry it asap. Oooh i like this. 12 calculate the mean and standard deviation of the three results in step 11.
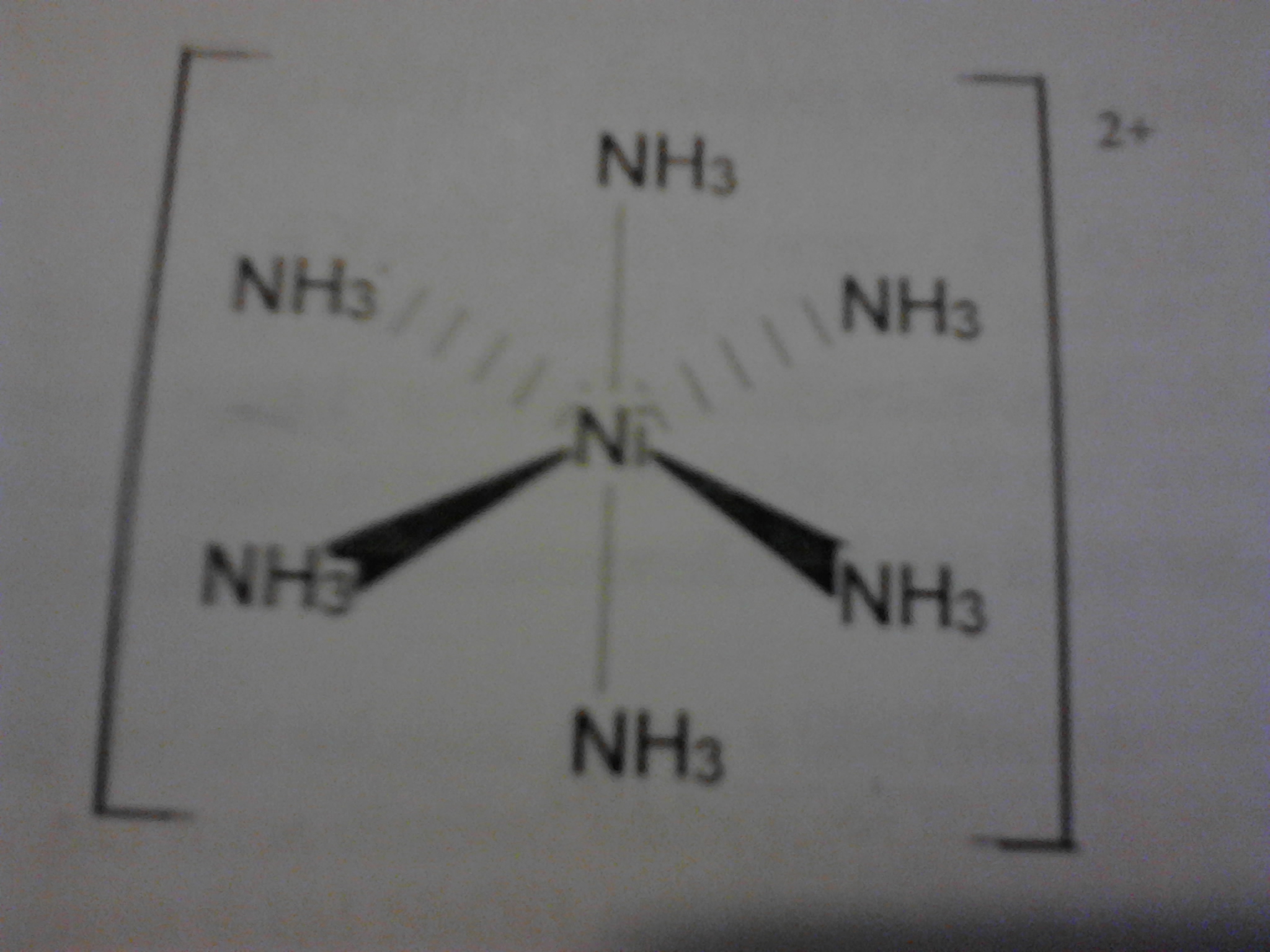
In the hood add 40. Hexamminenickel ii chloride analysis 3 9 calculate the millimoles of nh3 reacted with hcl according to reaction 3. The hexaammine nickel ii ion has six ammonia molecules surrounding the central nickel 2 ion in an octahedral arrangement the nickel ion has an oxidation state of 2 and each of the ammonia molecules is neutral so the overall charge on the complex ion is. A blue green precipitate was observed along with evolution of significant heat.
I don t know about the nickel complex but the copper easily gives up its ammonia for water so you have to be quick. 10 calculate the mass of nh3 molecular weight 17 03 g mol in the complex salt in each flask. Properties and structure ni nh 3 6 2 like all octahedral nickel ii complexes is paramagnetic with two unpaired electrons. The product ni nh 3 6 2 is called hexamminenickel ii ion.
Do not remove your beaker from the hood until the next step is completed. Hexaamminenickel chloride is the chemical compound with the formula ni nh 3 6 cl 2 it is the chloride salt of the metal ammine complex ni nh 3 6 2 the cation features six ammonia called ammines in coordination chemistry ligands attached to the nickel ii ion. Ml of 15 m ammonia pumping slowly and smoothly so that. 5 996g of nickel chloride hexahydrate was dissolved in 10ml of distilled water with stirring.
4 pour about 10 ml of aqueous ammonia into an erlenmeyer flask and set this in the ice to. Ive made this with copper sulfate before forming tetraaminecopper ii sulfate. Stir the reaction mixture until most of the solids dissolve. Aqueous ammonia was added slowly with vigorous stirring.
Synthesis of hexaamminenickel ii chloride click to order essay ethical pluralism essays 1 orstcr s biographical essays gubbins s siege of lucknow on the reparation of specimens and the whole forms u lit introduction to microscopic zoo ogy. Record the exact amount added to the precision limit of the top loading balance.