Hexaamminenickel Ii Chloride Colour
Pubchem substance id.
Hexaamminenickel ii chloride colour. Linear formula ni nh 3 6 cl 2. This corelates with what was seen in the uv vis. Iodide compounds are used in internal medicine. Hexaamminenickel 2 h18n6ni 2 cid 25199923 structure chemical names physical and chemical properties classification patents literature biological.
Visit chemicalbook to find more hexaamminenickel ii chloride 10534 88 0 information like chemical properties structure melting point boiling point density molecular formula molecular weight physical properties toxicity information customs codes. The nickel ions in the nicl 2 6h 2 o are hydrated when dissolved forming the green octahedral complex ni h 2 o 6 2. Seeing as we started with nicl 2 6h 2 o there is already chloride anions in the rxn. Hexamminenickel ii chloride analysis 3 9 calculate the millimoles of nh3 reacted with hcl according to reaction 3.
10 calculate the mass of nh3 molecular weight 17 03 g mol in the complex salt in each flask. You can also browse global suppliers vendor prices price manufacturers of hexaamminenickel ii chloride 10534 88 0. 255807 sigma aldrich hexaamminenickel ii chloride 99 999 cas number 10534 88 0. Absorption at 570 5 nm is the absorption of yellow colour and reflection of violet.
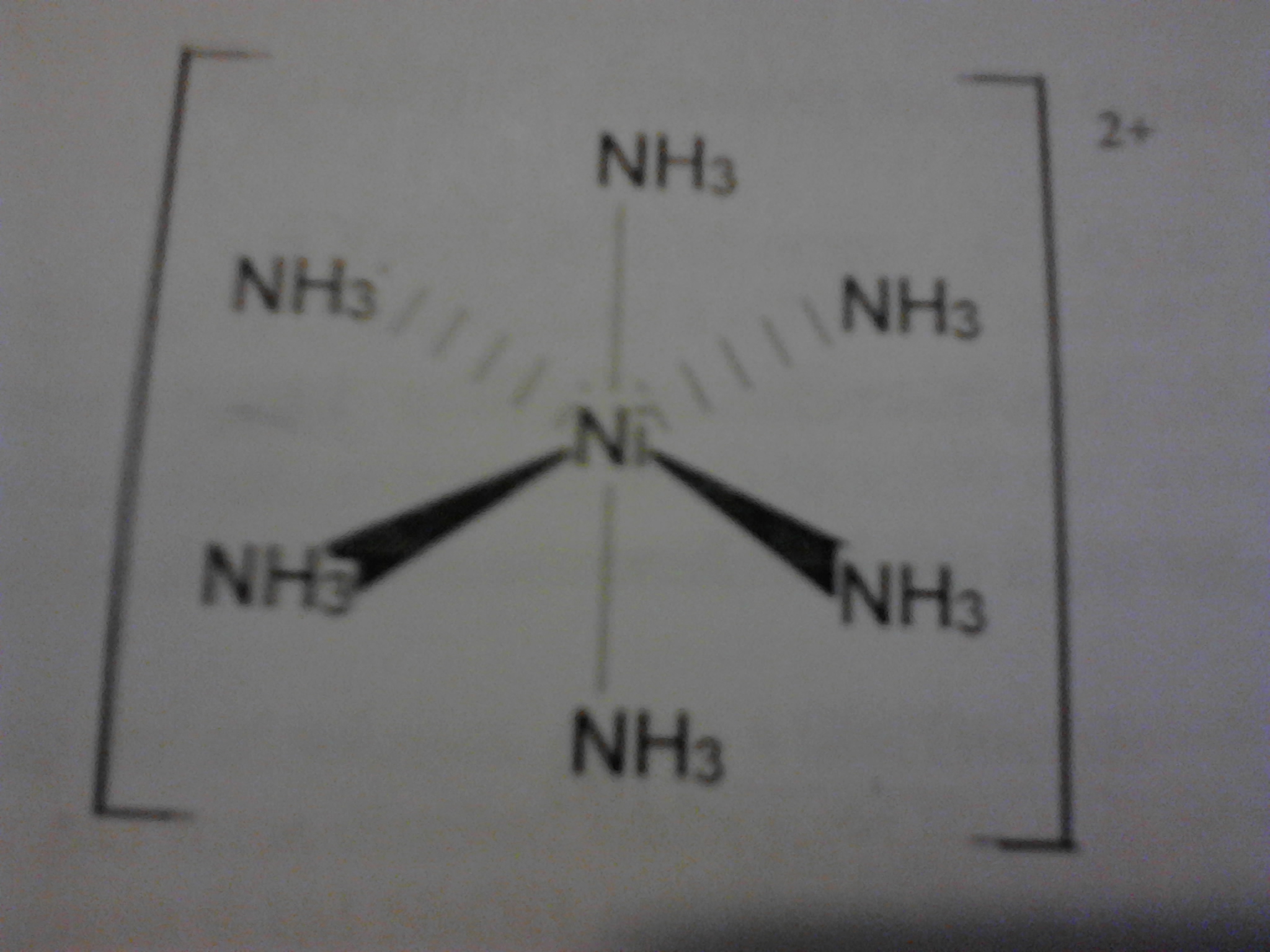
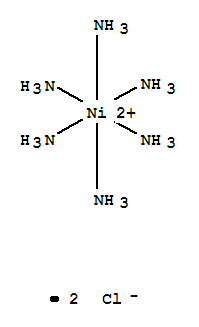
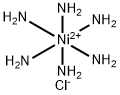
Molecular weight 231 78. Hexaamminenickel chloride is the chemical compound with the formula ni nh 3 6 cl 2 it is the chloride salt of the metal ammine complex ni nh 3 6 2 the cation features six ammonia called ammines in coordination chemistry ligands attached to the nickel ii ion. 12 calculate the mean and standard deviation of the three results in step 11. Hexaamminenickel ii chloride is generally immediately available in most volumes.
The hexammine complex is less soluble than the aqua ion and precipitates out as the chloride salt. 255807 hexaamminenickel ii chloride email this page to a friend. 11 calculate the percent by mass of nh3 in the three samples. The theoretical yields of hexaamminenickel ii chloride and tetraethylammonium tetrachloronickelate ii were 51 and 48 respectively.